Chet,
In response to your question, “1. Did the eTMF Technical Committee submit the vocabulary to NCI?”
No, the TC has NOT submitted any terms to NCI nor has CareLex submitted terms to NCI on behalf of the TC. CareLex has spent hundreds of hours working with NCI over more than a year to develop a controlled vocabulary at NCI. This work began prior to the TC’s formation and the body of work was first published at the Stanford National Center for Biomedical Ontology (Bioportal) for public review in 2012. View CareLex’s work here: http://bioportal.bioontology.org/ontologies/CARELEX?p=classes
Upon formation of the OASIS eTMF Standard TC, CareLex submitted and contributed the set of Category Terms, Annotation Properties, and Data Properties (collectively called Terms in this email) as a body of work for the OASIS TC to consider as a starting point, just as we contributed the technical specification to the TC for use as a starting point. CareLex has repeatedly informed the TC members that these terms were pulled together from a variety of sources and has noted such sources in our TC slides. Each source is also indicated in our work with the NCI, which publishes the terms for use by the global community.
While CareLex submitted the set of terms as a starting point for the TC, it is completely up to the collective TC to determine what the group will and will not use to achieve interoperability of the eTMF. Of course, the multiple rounds of Public Review will further inform the TC about the approach with the broadest appeal and use. The TC’s Metadata Workgroup spent time during three lengthy meetings reviewing and questioning the terms initially submitted and now seeks public comment regarding the list of Terms as well as the technical spec. Additionally, the OASIS eTMF TC is open to any and all who wish to join the TC’s efforts to establish a global standard for interoperability of the electronic trial master file.
CareLex’s work is open to public use and our aim is that the body of work resulting from OASIS eTMF Standard TC is free and open for public use. CareLex’s goal is information interoperability in the eTMF domain and for other health science domains in the future.
Best,
Jennifer Alpert Palchak, CEO
CareLex
Direct: 916-600-8388
www.carelex.org
This e-mail and any accompanying attachments are confidential. The information is intended solely for the use of the individual(s) to whom it is addressed. Any review, disclosure, copying, distribution, or use of this e-mail communications by others is strictly prohibited. If you are not the intended recipient, please notify us immediately by returning this message to the sender and delete all copies. Thank you for your cooperation.
From: etmf@lists.oasis-open.org [mailto:etmf@lists.oasis-open.org] On Behalf Of Chet Ensign
Sent: Wednesday, June 18, 2014 10:35 AM
To: etmf@lists.oasis-open.org
Cc: Jamie Clark; Carol Geyer; Paul Knight
Subject: [etmf] Request for clarification regarding -> Fwd: [etmf-comment] regarding Post: OASIS eTMF Standard Draft Approved
Members of the eTMF TC,
This is the second comment received today regarding a submission of the vocabulary to NCI. In reviewing the post Karin, linked, I note the statements that:
"The controlled vocabulary developed by the OASIS eTMF Standard Technical Committee was submitted to and published by the National Cancer Institute’s Enterprise Vocabulary Services and is available online at the NCI Thesaurus term repository at http://ncit.nci.nih.gov/." and "As part of the eTMF standardization effort toward a global ISO standard for clinical trial data exchange, The OASIS eTMF Standard TC will seek public review and comment on its recent draft publication which is available as a package for free download from OASIS at: https://www.oasis-open.org/committees/document.php?document_id=53345&wg_abbrev=etmf" which is followed by the heading "Comments and Public Contributions Welcome."
Regarding this second point, this does precisely what I asked you to avoid: it seeks comments on a working draft that can only lead to confusion when the actual public review is announced. I ask that you post a clarification to this blog posting explaining that OASIS will shortly issue the notice of the beginning of the formal public review period and that people should wait for that announcement and that official Public Review Draft of the specification before they provide comments.
Regarding the first point, I ask the TC to please answer the following questions:
1. Did the eTMF Technical Committee submit the vocabulary to NCI?
2. If the answer is yes, can you provide a link to the minutes where the motion was made and approved to make such a submission?
OASIS absolutely encourages liaisons and coordination. However, submission of working draft material is highly irregular. The work at this stage has no official standing, is not even preliminary much less final, and is not covered by the terms of the OASIS IPR Policy. Submission at this stage may, in fact, violate OASIS policy.
I would appreciate an answer to the above questions at your earliest convenience in order to determine if any additional steps need to be taken.
Thanks,
/chet
---------- Forwarded message ----------
From: Schneider, Karin [JRDUS] <KSchnei1@its.jnj.com>
Date: Wed, Jun 18, 2014 at 1:08 PM
Subject: [etmf-comment] regarding Post: OASIS eTMF Standard Draft Approved
To: "etmf-comment@lists.oasis-open.org" <etmf-comment@lists.oasis-open.org>
Hi, few comments regarding this post:
OASIS eTMF Standard Draft Approved
· There is a 45-day public review period but I find the information on NCI as if it was final, why?
· The source is identified as “CareLex”, I thought this is an independent Oasis initiative, am I wrong?
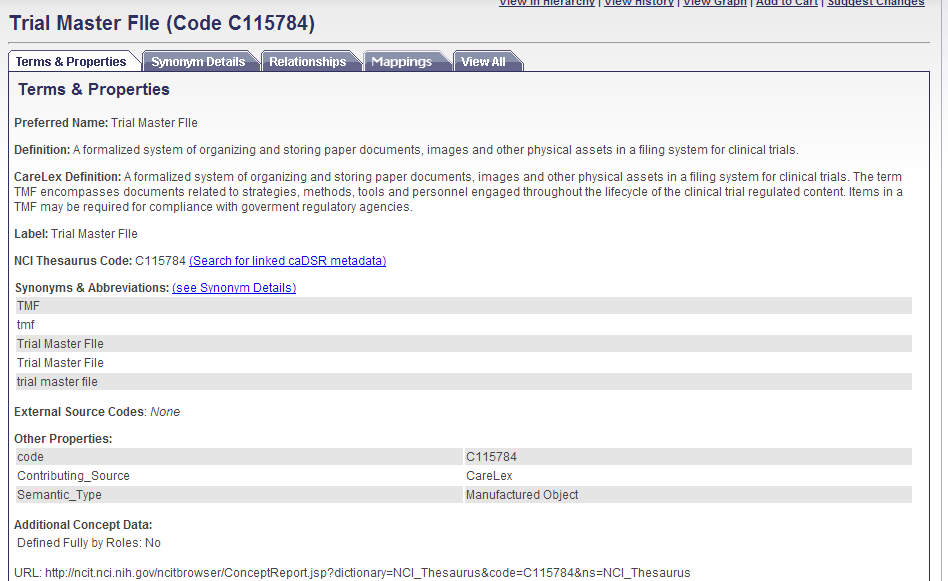
· It states: Support for the TMF Reference model — OASIS eTMF Standard controlled vocabulary terms were reviewed by members of the TMF Reference Model community to provide a cross-reference between terms in the OASIS eTMF Standard controlled vocabulary and terms in the TMF Reference Model. Published in the OASIS eTMF Standard controlled vocabulary, the cross-referencing resource is designed to provide a path forward to groups who may have implemented the TMF Reference model terms and are interested in migrating to the OASIS eTMF Standard for existing or new clinical studies. I am part of the TMF Ref model community and would be surprised if my fellow members approved the term below (example) which has an altered definition and is misspelled.
· What is the level of no NCI governance that was applied to approve new terminology and position its use accurately?
Thank you,
Karin Schneider
TMF Reference Model SC team member
--
/chet
----------------
Chet Ensign
Director of Standards Development and TC Administration
OASIS: Advancing open standards for the information society
http://www.oasis-open.org
Primary: +1 973-996-2298
Mobile: +1 201-341-1393
Check your work using the Support Request Submission Checklist at http://www.oasis-open.org/committees/download.php/47248/tc-admin-submission-checklist.html
TC Administration information and support is available at http://www.oasis-open.org/resources/tcadmin
Follow OASIS on:
LinkedIn: http://linkd.in/OASISopen
Twitter: http://twitter.com/OASISopen
Facebook: http://facebook.com/oasis.open